
The Guideline for the prophylactic use of Rh D immunoglobulin in pregnancy care provides recommendations and expert opinion points for health professionals on the appropriate use and timing of:
- pathology tests to identify maternal and fetal Rh D status
- prophylactic Rh D immunoglobulin in Rh D negative pregnant women
The guideline was a joint project between the NBA and Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG) and replaces the Guideline for the prophylactic use of Rh D immunoglobulin in pregnancy care 2021 and Guidelines on the Prophylactic Use of Rh D Immunoglobulin (Anti-D) in Obstetrics in 2003.
The recommendation on the use of non-invasive prenatal testing (NIPT) for fetal RHD status is based on scientific evidence and consensus among clinical experts; it is not a policy statement on funding and supply arrangements for the national provisions of NIPT. The existing supply arrangements and MBS funding arrangements for the national provision of NIPT to determine fetal RHD status are not specifically addressed in the Guideline.
Background
In 2016, the NBA and RANZCOG agreed on the need to update the 2003 guideline while maintaining the policy intent for an antenatal prophylaxis program. The update was published on the NBA website in 2021. Shortly after publication the literature searches were updated, and relevant evidence incorporated into the guideline. The guideline update was overseen by an expert reference group (ERG) made up of clinical experts and a consumer representative. The NBA gratefully acknowledges the significant contribution made by members of the ERG. The Guideline was published on 6 March 2024 in electronic format and on MAGICapp.
The Guideline and other resources can be accessed by clicking on the links below.

Resources

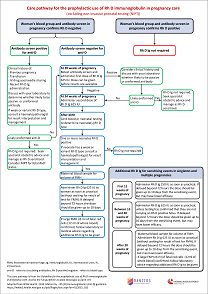

- the guideline recommendations and expert opinion points
- use and timing of pathology testing table
- administration of Rh D immunoprophylaxis table
- adminsitration of Rh D immunoglobulin for sensitising event immunoprophylaxis table
- care pathways that outline the timing of pathology testing and administration of Rh D immunoglobulin
Technical report on the methodology and findings of the evidence reviews
Volume 1 of the technical report contains background information and the systematic review results.
Maintenance of the Guideline
The evidence used to develop recommendations in the guideline was last updated in September 2021. To keep the guideline current the ERG will prioritise the recommendations and expert opinion points and determine the frequency of future literature searches and surveillance. Any updates to the guideline will be made in MAGICapp.
Education resources
BloodSafe eLearning have developed a free online course for health professionals involved in caring for Rh D negative pregnant women. The course can be accessed at RhD Immunoglobulin BloodSafe eLearning Australia.
College and Society endorsements
The NBA greatly appreciates and acknowledges the input received from the clinical community in producing the guideline. We are pleased to acknowledge that the following organisations have formally endorsed the guideline:
- The Royal Australian and New Zealand College of Obstetricians and Gynaecologists (RANZCOG)
For more information
To provide feedback and inform future reviews of this guideline, please send any comments on its content or implementation, or on the accompanying materials, to the project manager at:
- Email guidelines@blood.gov.au
- Mail:
Patient Blood Management Guidelines
National Blood Authority
Locked Bag 8430
Canberra ACT 2601




