- Overview
- National Immunoglobulin Governance Advisory Committee (NIGAC)
- Immunoglobulin Specialist Working Groups (SWGs)
- Jurisdictional Immunoglobulin Performance Improvement Group (JIPI)
- Jurisdictional Immunoglobulin Interest Groups (JIIGs)
- National Immunoglobulin Interest Group (NIIG)
- How to get involved
- Immunoglobulin (Ig main menu and landing page)
Overview
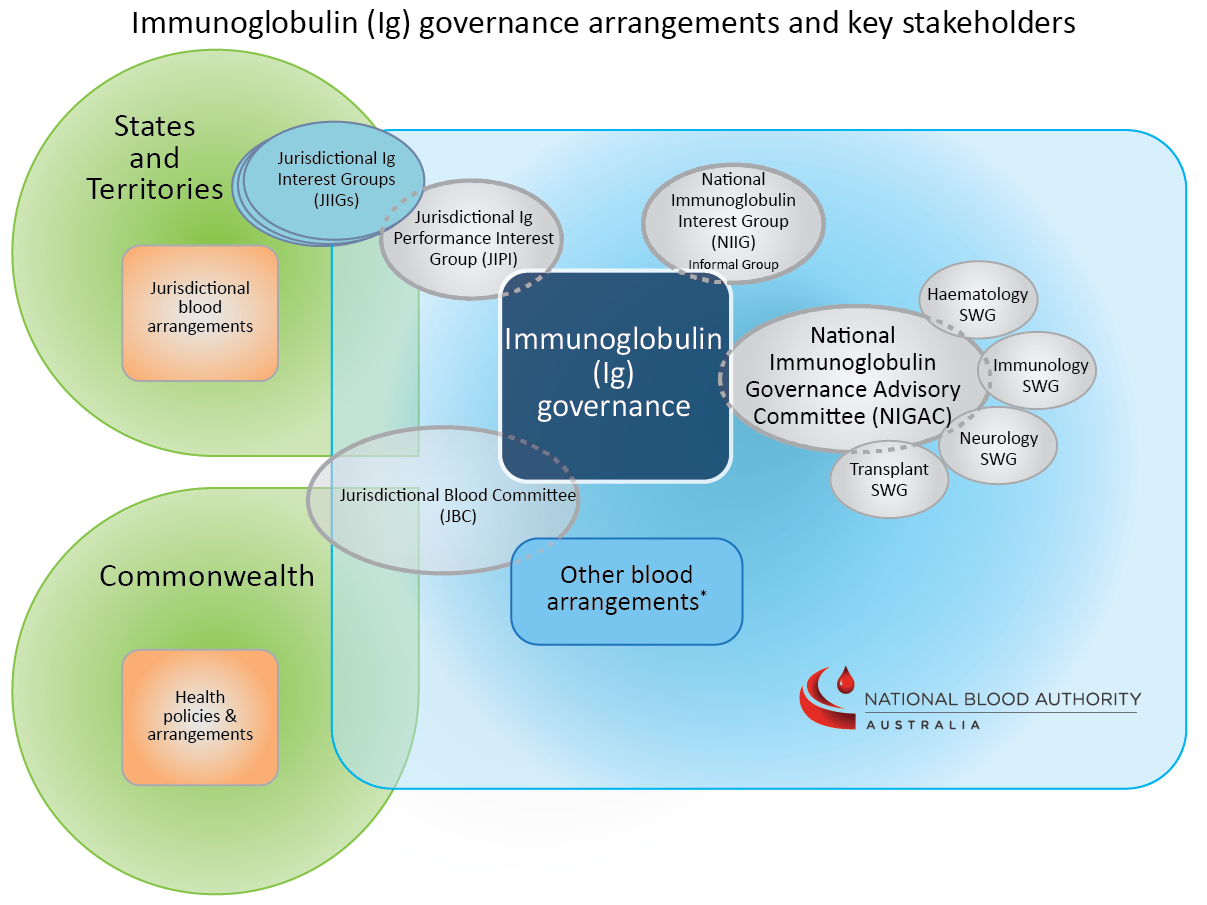
A network of committees and interest groups provide formal and informal forums for informing the work of the national immunoglobulin (Ig) governance program. These committees interact with national health policy committees and forums, as appropriate.
The principal NBA Ig governance committee is the National Immunoglobulin Governance Advisory Committee (NIGAC). Four immunoglobulin specialist working groups (SWGs) provide expert advice and comment to the NBA in the areas of haematology, immunology, neurology and transplantation.
The informal Jurisdictional Immunoglobulin Performance Improvement (JIPI) Group provides a forum for the NBA and jurisdictions to share information, and coordinate and discuss immunoglobulin governance-related activities and initiatives, complementing the work of the jurisdictions’ own immunoglobulin interest groups (known generically as the Jurisdictional Immunoglobulin Interest Groups, or JIIGs).
Individuals involved in any aspect of Ig use and management (from medical professionals to patients and carers) who would like to keep abreast of, and potentially contribute to, discussions on relevant topics are encouraged to join the National Immunoglobulin Interest Group (NIIG). Members of the NIIG receive Ig Program Updates, a quarterly snapshot of the NBA’s current immunoglobulin-related work program and priorities. They may also be invited to provide comment on discrete issues on an ad-hoc basis, as required.
Below: Illustrative diagram of the immunoglobulin governance arrangements and network of committees
*Find more information on key bodies in the Australian blood sector here
National Immunoglobulin Governance Advisory Committee (NIGAC)
Overview
The NIGAC is a statutory committee and the peak immunoglobulin governance committee. Established by the NBA Chief Executive under section 38 of the National Blood Authority Act 2003, it provides strategic advice and recommendations to the NBA on all aspects of the Immunoglobulin Governance Program. Members are formally appointed by the NBA.
Purpose and Terms of Reference
NIGAC’s role is to provide advice and make recommendations to the NBA to support the Ig Governance Program, which seeks to ensure that Ig product use and management reflects appropriate clinical practice and represents efficient, effective and ethical expenditure of government funds. NIGAC’s key tasks include making recommendations and providing guidance to:
- evolve the Criteria for the Clinical Use of Immunoglobulin in Australia (the Criteria), which govern access to Ig funded under the national blood arrangements
- ensure access to and management of nationally-funded Ig is fair and equitable, as set out in the Criteria and the National Policy: Access to Government-funded Immunoglobulin Products in Australia (National Policy)
- improve all aspects of Ig management, access and governance arrangements through data analysis, research and education.
For an overview of each of these elements, with links to more detailed information, see the Ig landing page at: Immunoglobulin.
NIGAC members also play a critical leadership role, acting as ‘champions’ for the Program.
NIGAC Terms of Reference are available here ![]() pdf (719.95 KB) or
pdf (719.95 KB) or ![]() docx (854.49 KB).
docx (854.49 KB).
Membership
Members are appointed by the NBA Chief Executive on the basis of expertise and experience, with membership renewed through a rolling membership review process. Initial appointments are for three years, with a maximum term of membership of eight years.
All key Ig stakeholder groups are represented, as illustrated below. (Note: this information is also included in the NIGAC Terms of Reference ![]() pdf (719.95 KB) or
pdf (719.95 KB) or ![]() docx (854.49 KB))
docx (854.49 KB))
|
Role |
Member details |
|
|---|---|---|
|
Chair |
Professor Robert Moulds Medical Advisor, Therapeutic Guidelines Ltd; Emeritus Professor of Medicine, Fiji National University |
|
|
Medical Specialist Representative, Neurology |
Specialist Working Group Chairs |
Associate Professor Stephen Reddel Staff Specialist Neurologist Concord Repatriation General Hospital, NSW |
|
Medical Specialist Representative, Haematology |
Associate Professor Dipti Talaulikar Senior staff Specialist, Clinical Haematologist, Canberra Hospital; Associate Professor, College of Medicine, Biology and Environment, Australian National University, ACT |
|
|
Medical Specialist Representative, Immunology |
Dr Melanie Wong Senior Staff Specialist Immunologist and Immunopathologist Director of Immunology Laboratory; Head, Immunodeficiency Service, Department of Allergy and Immunology The Children’s Hospital at Westmead, Sydney, NSW |
|
|
Medical Specialist Representative, Transplantation |
Associate Professor Kate Wyburn Senior Staff Specialist Nephrology Royal Prince Alfred Hospital, Sydney, NSW
|
|
|
Consumer Representatives (Two positions) |
Mr Adam Friederich Deputy Board Chair Immune Deficiencies Foundation Australia |
|
|
Mr Mark Kunach Chair, GBS Association of NSW
|
||
|
Dispenser Representative |
Ms Annette Le Viellez Senior Scientist in Charge Transfusion Medicine, PathWest South-East Division WA
|
|
|
Epidemiologist |
Dr Surendra Karki Research Fellow/Epidemiologist, Research and Development, Australian Red Cross Lifeblood; Conjoint Lecturer, School of Public Health and Community Medicine, the University of New South Wales (UNSW), Sydney, NSW
|
|
|
Health Economist |
Associate Professor Silva Zavarsek Associate Professor Health Technology Assessment Centre for Population Health Research, Monash Business School, Deakin University, VIC |
|
|
Nurse Representatives (Two positions) |
Ms Noelene Davies State IVIg Nurse, Advanced Clinical Practice Consultant Royal Adelaide Hospital; Australian Red Cross Lifeblood, SA |
|
|
Ms Rachael Dunn Clinical Nurse Specialist Immunology Perth Children’s Hospital, WA |
||
|
Commonwealth Representative |
Dr Neil Everest Advisor, Technology Assessment and Access Division |
|
|
Large Jurisdiction Representative |
Dr Katherine Ong Medical Adviser, Specialty Programs Health and Wellbeing Division Department of Health and Human Services, Victoria |
|
|
Small Jurisdiction Representative |
Ms Carolyn Duck ACT Blood Counts Program Manager / Senior Policy Officer Blood and Blood Products Canberra Health Services, ACT |
|
|
NBA Representatives (Two positions) |
Mr John Cahill Chief Executive National Blood Authority |
|
|
Dr Anna Peatt Deputy Chief Executive National Blood Authority |
||
|
Authoriser – Observer (Two positions) |
Dr Joanne Pink Chief Medical Officer and Executive Director Clinical Services and Research Australian Red Cross Lifeblood |
|
|
Dr Janet Wong Australian Red Cross Lifeblood
|
||
A list of current NIGAC members is available here ![]() National Immunoglobulin Governance Advisory Committee (NIGAC) Members, August 2021 (pdf)or
National Immunoglobulin Governance Advisory Committee (NIGAC) Members, August 2021 (pdf)or ![]() National Immunoglobulin Governance Advisory Committee (NIGAC) Members, August 2021 (doc).
National Immunoglobulin Governance Advisory Committee (NIGAC) Members, August 2021 (doc).
Interested in joining NIGAC? See: How to get involved.
Meetings
NIGAC meets up to four times per year. Usually, one of these meetings is face-to-face, with others held via video or teleconference. See Ig Program Updates for an overview of NIGAC’s current work program.
Immunoglobulin Specialist Working Groups (SWGs)
Overview
Four immunoglobulin SWGs provide expert advice and comment to the NBA in the areas of haematology, immunology, neurology and transplantation. Representation is sought from additional specialty areas (e.g. rheumatology, dermatology) on an ad hoc basis, as required.
Members are formally appointed by the NBA Chief Executive on the basis of expertise and experience, with the Chair of each SWG formally representing their group’s position as a member of NIGAC.
Purpose and Terms of Reference
The key role of each SWGs is to:
- provide specialist feedback, clinical advice and make recommendations in relation to the Criteria for the Clinical Use of Immunoglobulin in Australia
- define the relative prioritisation of each indication for eligibility during times of product shortage.
- provide specialist insight and advice to inform continuous improvement activities, including through:
- conducting data and report reviews and recommending, developing and commissioning clinical treatment guidelines, research projects, consensus statements and other knowledge development initiatives
- identifying and recommending improvements to education and training initiatives
- providing advice and feedback on improvements to the national online system used to request, authorise and manage Ig in line with the Criteria: BloodSTAR.
For an overview of each of these elements, with links to more detailed information, see the Ig landing page at: Immunoglobulin.
SWG members also play a critical leadership role, acting as ‘champions’ and facilitators for the Program.
SWG Terms of Reference are available here ![]() pdf (219.07 KB) or
pdf (219.07 KB) or ![]() docx (50.07 KB) .
docx (50.07 KB) .
Membership
Each SWG includes a minimum of five medical specialist representatives, with the qualifications and clinical expertise that enable them to consider the clinical appropriateness, safety and cost-effectiveness of Ig, including comparative outcomes of different therapies. The Australian Red Cross LifeBlood is an observer on all SWGs; a representative attends meetings to participate in discussion, but does not take part in decision-making processes.
Members are appointed by the NBA Chief Executive on the basis of expertise and experience. Consideration is also given to ensuring diversity; where relevant, committees seek to include members representing both adult and paediatric specialties, from a mix of jurisdictions, including both metropolitan and rural/remote practices, where possible.
Membership is renewed through a rolling membership review process, with initial appointments of three years.
Interested in joining an SWG? See: How to get involved.
A list of current SWG members is available here ![]() pdf (373.92 KB) or
pdf (373.92 KB) or ![]() docx (50.7 KB).
docx (50.7 KB).
Meetings
SWGs meet up to 4 times a year, as required, with further out-of-session discussion as needed between meetings to progress issues. Usually, one meeting per year is scheduled to be face to face. See Ig Program Updates for an overview of the SWGs’ current work programs.
Jurisdictional Immunoglobulin Performance Improvement (JIPI) Group
Overview
The Jurisdictional Immunoglobulin Performance Improvement (JIPI) Group was established in 2020 as an informal group to support the NBA’s Ig performance improvement work, which seeks to improve the prescription, use and management of government-funded Ig products accessed through the National Ig Governance Program. The JIPI Group replaced the Immunoglobulin Health Department Liaison Group, which was set up in 2014 to support the development and implementation of the Ig Governance Program.
The JIPI Group provides a link and a forum for discussion between the people responsible for implementing the Ig governance arrangements within jurisdictions, and the Ig Governance Program. Members are nominated by each jurisdiction’s health department, and are usually members of their respective jurisdiction’s own Jurisdictional Ig Interest Group (JIIG), or equivalent.
Purpose
JIPI aims to facilitate and support an integrated, nationally-consistent and collaborative approach to all activities, projects and work programs under the Immunoglobulin Governance Performance Improvement Strategy (the Strategy) with key aims being to:
- support the development and implementation of the Strategy through activities to enhance relevant data collection, sharing, analysis, benchmarking, reporting and governance activities conducted by the NBA and other relevant parties
- support the further development of compliance elements of the Strategy as appropriate within the public and private segments of the health sector.
Membership
Membership comprises representatives from each State, Territory and Commonwealth government, as well as representatives from the NBA. To be able to participate in this informal group, members must have a strong interest in Ig governance, a broad knowledge of healthcare, and be able to represent their jurisdiction to support and facilitate performance improvement activities.
Meetings
Meetings are held on an ‘as needs’ basis by tele- or videoconference. As the aim of the group is to provide a forum for sharing ideas and experience, there is often additional communication and coordination between meetings. See Ig Program Updates for information about issues recently discussed.
Jurisdictional Immunoglobulin Interest Groups (JIIGs)
Overview
As the name suggests, Jurisdictional Ig Interest Groups (JIIGs) are created at a jurisdictional level, by individual state or territory health departments. They are not an NBA committee or group, but are described here because they are an integral part of the network of committees responsible for immunoglobulin governance and management in Australia. Engaging with the JIIGs, mostly through JIPI, benefits both groups by facilitating engagement and communication between the policy makers and those responsible for its implementation at a jurisdictional and facility level. This is achieved by, for example:
- helping to identify, communicate and resolve issues relating to the governance arrangements for Ig, including the application of the Criteria
- facilitating the development and implementation of program improvement activities.
Membership
Membership differs across the groups, but generally includes representatives with an interest in Ig use and governance from the jurisdiction’s health department; specialists in the fields of immunology, neurology, haematology and transplantation; the Australia Red Cross Lifeblood; nurses; medical scientists; and patients.
Interested in joining your local JIIG? See: How to get involved.
National Immunoglobulin Interest Group (NIIGs)
Overview and purpose
The National Immunoglobulin Interest Group (NIIG) is an informal subscription-based group for anyone interested or involved in any aspect of Ig use and management, from medical professionals, policy-makers and administrators, to patients and their carers.
Members of the NIIG receive the NBA’s quarterly Ig Program Updates. They may also be invited to informally discuss and comment on individual issues as they arise, including, for example:
- Ig governance policy arrangements
- Ig Governance Program implementation activities
- educational material and resources on immunoglobulin
- BloodSTAR functionality and useability
- program areas where improvements could be made
- program enhancement proposals to determine if they are fit for purpose.
Or, they may be invited to make more specific contributions to specific projects, such as:
- providing advice and feedback on system changes and enhancements to BloodSTAR and participate in User Acceptance Testing when there is a major release of BloodSTAR
- providing feedback on educational material and resources being developed for prescribers of Ig and patients.
Membership
Interested in joining NIIG? See: How to get involved.
How to get involved
The Ig Governance Program relies on the input and feedback of stakeholders. There are numerous ways to get involved:
Committee membership
- NIGAC – NIGAC membership is currently closed. NBA will begin seeking formal expressions of interest for membership from suitably qualified individuals in mid-2021, with a view to appointments being confirmed in late 2021.
To keep informed, check this page and the Ig Program Updates page for updates, or subscribe to Ig Program Updates and the National Immunoglobulin Interest Group (details below).
- SWGs – SWG membership is currently closed. NBA will begin seeking formal expressions of interest to join its SWGs in mid-2021 with a view to appointments being confirmed in late 2021.
To keep informed, check this page and the Ig Program Updates page for updates, or subscribe to Ig Program Updates and the National Immunoglobulin Interest Group (details below).
- JIPI Group – membership is by invitation only.
- JIIG Group – Membership is determined by each jurisdiction.
- NIIG – Join at any time by subscribing to Ig Program Updates and the National Immunoglobulin Interest Group through BloodPortal. To do this:
- create a BloodPortal account (if required)
- log in
- from the home page, click on ‘My Subscriptions’ and follow the instructions.
Questions about membership? Please contact Iggovernance@blood.gov.au. Please include the key points in the message subject line, be as clear as you can, and remember to include your contact details.
Feedback and Questions
Questions about committee or interest group membership, and general questions or feedback on issues of Ig governance may be directed to: Iggovernance@blood.gov.au, or call 13 000 BLOOD (13 000 25663).
Feedback specifically relating to BloodSTAR should be directed to: support@blood.gov.au, or call the Blood Operations Centre on 13 000 BLOOD (13 000 25663).
When emailing, please include the key points in the message subject line, be as clear as you can, and remember to include your contact details.