In This February 2017 Issue:
- Go-live update: BloodSTAR now live in all jurisdictions except NSW
- BloodSTAR system update: New functions now available
- Intragam 10 Transition: What you need to know now
- Blood Service Contacts: Important contact numbers for authorisations
- For further information and how to sign up for newsletters
- Download BloodSTAR News -
 February 2017 pdf (146.48 KB)
February 2017 pdf (146.48 KB)
Go-live update: BloodSTAR Live in all jurisdictions, except NSW
BloodSTAR is now live in all jurisdictions, except NSW, with:
- over 7,670 patients with active authorisations, and
- approximately 5,660 registered users accessing BloodSTAR as either Authorisers, Medical Officers, Nurses or Facility Administrators (in addition to Dispensers using BloodNET).
During January there were 1,071 initial authorisation requests and 9,737 dispense episodes of IVIg in BloodSTAR nationally.
During December there were 1,154 initial authorisation requests and 9,472 dispense episodes of IVIg in BloodSTAR nationally.
BloodSTAR system update: New functions now available
The NBA continues to work with users to ensure the system is fit for purpose. On 22 January we released and updated version of BloodSTAR (version 2.4) with new functions and enhancements. The new functions are:
Nurses/Midwives/Admin Support
Planning sheets - Display on 4:3 ratio screens has been improved making it easier to add a patient to a planning sheet.
Facility Administrator
Access requests - Fixed an issue where users could enter more than 20 characters in the phone, mobile or fax fields which would generate an error if a facility administrator attempted to approve access.
Authoriser/Authoriser Administrators
Review assessment - A patient’s current authorised dose and weight is now visible to Authorisers at review and dose change requests.
Initial authorisation assessment - Auto population of requesting doctor in Treating Medical Specialist field has been removed to encourage entering of the correct treating medical specialist details.
Medical Officers
Improving access to current patient information – Medical officers now have the ability to see their patient’s most recent Ig dose and weight when completing a review. They are displayed at the top of the screen with your patient’s details.
Initial authorisation request – Removal of the automatic population of requesting doctor in Treating Medical Specialist field to ensure the user enters the correct specialist at the initial authorisation request stage.
Medical Officers, Nurses/Midwives, Admin Support, Dispensers and Authorisers
Other updates include:
- Preparation work for transition to Intragam 10 and future product management - As part of the upcoming transition from Intragam P to Intragam 10, the existing product management functionality has been revamped to cater for the introduction of Intragam 10 as well as other future product changes. Extensive testing has been undertaken to ensure minimal disruption to ongoing patient care upon transition. BloodSTAR will automatically transition patients to Intragam 10 according to transition dates. Further transition details can be found on the NBA website https://www.blood.gov.au/plasma-and-recombinant-product-procurement
- General security updates - A range of security improvements have been implemented throughout BloodSTAR to strengthen controls, secure system messages, obscure browsers and update database configuration that will further improve protection against malicious threats.
- Minor bug repairs and performance improvements to BloodSTAR.
The next BloodSTAR system update (2.5) is in development with a focus on dosing and authorisation improvements.
Intragam 10 Transition
BloodSTAR and BloodNet users would have received an email detailing the transition of the domestically produced intravenous immunoglobulin (IVIg) product Intragam P to Intragam 10.
From March 2017, the 10% concentration Intragam 10 will be introduced and eventually replace the current 6% concentration Intragam P. Intragam 10 will provide lower infusion volumes for patients compared with the same immunoglobulin dose of Intragam P. The core plasma fractionating manufacturing process is the same for both products.
What do you need to know now?
1. In all states and territories except NSW:
New patients
From 1 March 2017, new patients with conditions for which domestic IVIg is allocated will be allocated to receive Intragam 10 in BloodSTAR.
Existing patients who are currently authorised to receive Intragam P
a. From 1 March 2017, existing patients for whom an authorisation request is submitted in BloodSTAR will be allocated to receive Intragam 10
b. All remaining existing patients will be transitioned to Intragam 10 as national inventories of Intragam P are reduced. BloodSTAR will automatically update dose calculations when transition occurs.
2. In NSW only:
New patients
From 1 March 2017, new patients with conditions for which domestic IVIg is allocated will be allocated to receive Intragam 10.
Existing patients who are currently authorised to receive Intragam P
Will be transitioned to Intragam 10 as national inventories of Intragam P are reduced.
Please note: Transition to from Intragam P to Intragam 10 in NSW will be managed outside of BloodSTAR by the Blood Service existing processes.
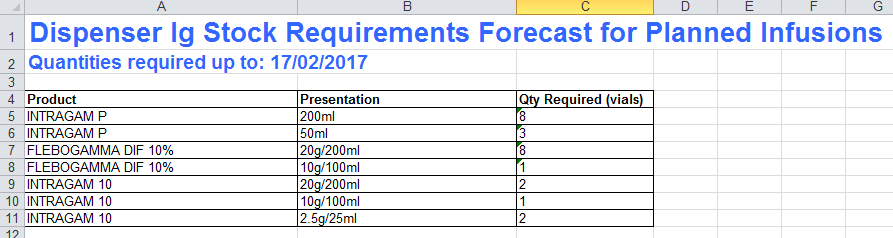
Dispenser Inventory Management
Remember to use the Ig Stock Requirements Forecast Report in BloodNET to help decide how much Intragam 10 and Intragam P you will need in your inventory during transition. Consider adding a small amount of Intragam 10 to your stock order for new authorisations in the first month.
For more information, including fact sheets for Health Professionals and Patients, please go to our plasma and recombinant product procurement page.
Blood Service Contacts
The Blood Service contact details for BloodSTAR authorisations differ depending upon the jurisdiction and time of day. When a prescriber submits an emergency authorisation request, they are prompted to call the Blood Service and are presented with the contact numbers for each jurisdiction. Please ensure you call the number relevant to your jurisdiction only.
The numbers below also apply to dispensers when contacting the Blood Service for BloodSTAR-related enquiries.
Please note: the after-hours numbers are based on local time. For example users in the ACT will call QLD after 4:30pm ACT time.
|
JURISDICTION |
FOR URGENT ENQUIRIES 8:30am to 4:30pm local time |
AFTER HOURS PHONE NUMBER 4:30pm to 8:30am local time |
FAX NUMBER: |
|
ACT |
1300 478 348 |
07 3838 9010 |
02 9234 2050 |
|
NSW |
1300 478 348 |
1300 478 348 |
02 9234 2050 |
|
NT |
08 8928 5116 |
03 9694 0200 |
08 8927 5461 |
|
QLD |
07 3838 9223 |
07 3838 9010 |
07 3838 9421 07 3838 9400 (After Hours) |
|
SA |
08 8112 1341 |
08 8223 6090 |
08 8223 5833 08 8232 5741 (After Hours) |
|
TAS |
03 6215 4122 |
03 9694 0200 |
03 6215 4197 |
|
VIC |
03 9694 0200 |
03 9694 0200 |
03 9694 0245 |
|
WA |
08 9421 2377 |
08 9325 3030 |
08 9221 1215 |
Please remember that the Ig request forms that are available online are for NSW only. All other jurisdictions must use BloodSTAR. Please do not fax a form to the NSW number if your patient is receiving treatment in a jurisdiction other than NSW.
For further information
Further information on BloodSTAR is available online here or by contacting the NBA on 13 000 BLOOD (13 000 25663) or support@blood.gov.au
Previous editions
To read or download previous editions of BloodSTAR News, click on the relevant link below:


