
In this July 2017 issue:
- BloodSTAR update: BloodSTAR numbers from live jurisdictions
- BloodSTAR system update: New functions now available
- Dosing Changes: Changes for some conditions
- User tip
- Criteria changes: Where are we up to
- For further information and how to sign up for newsletters
- Download BloodSTAR News
 Pdf (302.48 KB)
Pdf (302.48 KB)
BloodSTAR update: BloodSTAR numbers from live jurisdictions
The authorisation and user numbers in BloodSTAR live jurisdictions to this month:
- Over 7,700 patients with active authorisations, and
- Approximately 6,700 registered users accessing BloodSTAR as either Authorisers, Medical Officers, Nurses or Facility Administrators (in addition to Dispensers using BloodNET).
During April there were 1,194 initial authorisation requests and 10,284 dispense episodes of IVIg in BloodSTAR nationally.
BloodSTAR system update: New functions now available
The NBA continues to work with users to ensure the system is fit for purpose. On 2 April we released an updated version of BloodSTAR (version 2.5) with new functions and enhancements. The new functions are:
Authoriser/Authoriser Administrators
Treatment plan preview for Authorisers: a new link called “Preview Treatment Plan” is available for authorisers in the Assessment screen; and in certain circumstances, based on the assessment outcome; the link shall be displayed on the Dose Change Assessment, Assess Additional Dose, Authorisation Assessment and Continuing Authorisation Assessment screens.
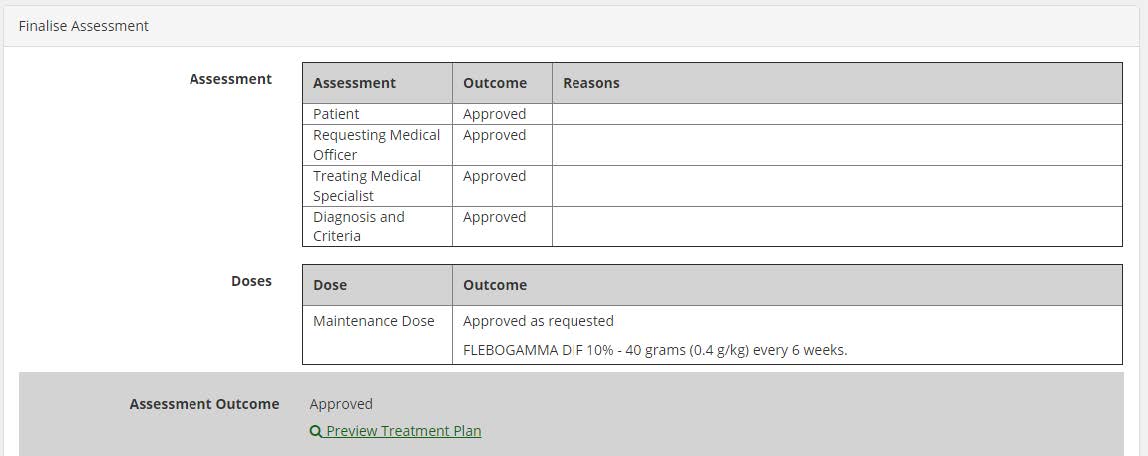
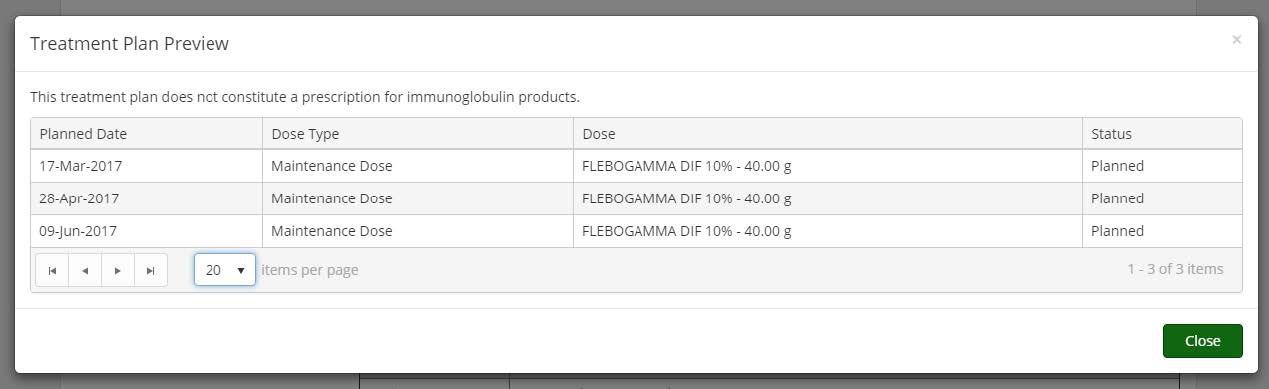
Treatment Plan Preview:
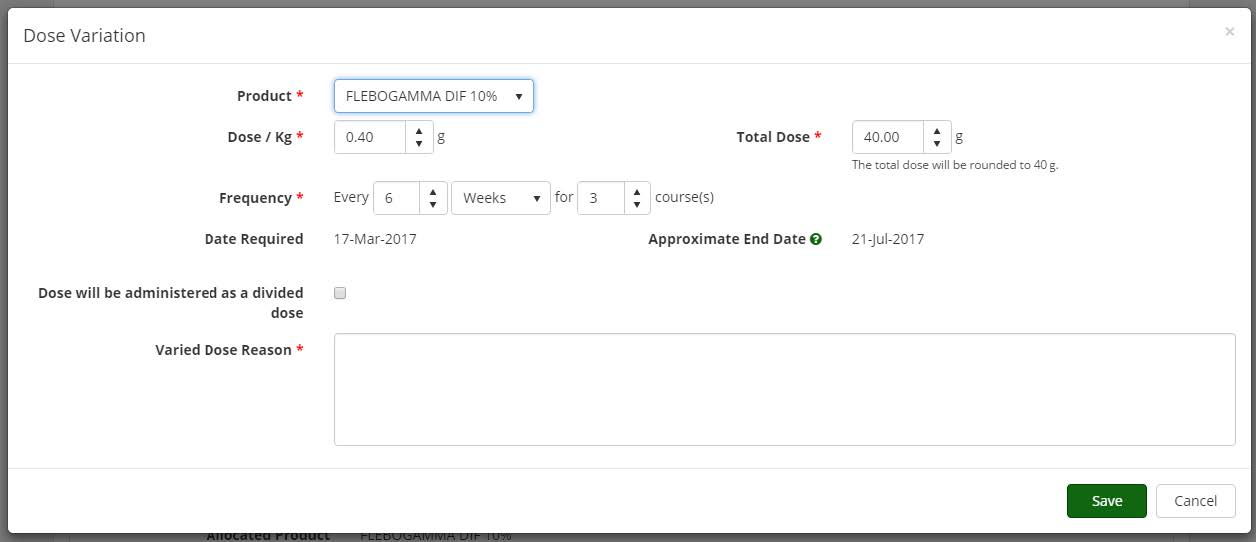
Authoriser’s ability to edit an authorisation Edit Dose: provides Authorisers the ability to edit an authorisation if an error is made during the authorisation process. The system notifies the patient, dispensing facility, Treating Medical Specialist and Requesting Medical Officer when a modification of an authorisation is made by the Authoriser.
Medical Officers
Dosing information – the “Dose Notes” field will be renamed to “Comments” for all type of doses.
Loading dose frequency – the “Once only” box will be ticked by default when a Medical Officer selects One off dose, Induction Dose or Loading Dose for an unspecified condition. And it will be un-ticked by default when a Medical Officer selects Maintenance Dose for an unspecified condition.
Nominating your own division – the medical officer will have the ability to determine the quantity for each divided dose; not just have to manipulate the total dose to get the dosing they want. This will affect initial assessment, continuation, dose change and additional dose request processes.
Removing default medical officer at review – the Reviewing Medical Officer section will now ask for the “Name of medical officer completing the review” instead of defaulting to the Treating Medical Specialist at review.
BloodSTAR dosing and prescribing changes
Following user feedback, BloodSTAR has been updated to allow the following prescribing and dosing regimens for patients authorised in the following listed transplantation, neurology, haematology and immunology conditions for the indications described below.
Neurology
| Condition | Indication | Change |
| Chronic inflammatory demyelinating polyneuropathy (CIDP) |
|
An additional induction dose is now permitted if the patient has acute deterioration / relapse while on Ig therapy. Consultation with a neurologist is required. |
| Multifocal motor neuropathy (MMN) |
|
|
| Myasthenia gravis (MG) |
|
|
| Polymyositis (PM) and Dermatomyositis (DM) |
|
Haematology
| Condition | Indication | Change |
| Immune Thrombocytopenic Purpura (ITP) – adult |
|
An additional dose request is now permitted for the remaining part of the initial dose if the full dose of 2 g/kg has not yet been prescribed. |
| Immune Thrombocytopenic Purpura (ITP) – children 15 years and younger |
|
The remaining part of the initial dose is now permitted if the full dose of 2 g/kg has not yet been prescribed. This provides consistency with the approach in ITP - adult. |
Immunology
| Condition | Indications | Change |
| Kawasaki disease |
|
Kawasaki disease can now be requested by an intensivist in addition to the following specialties: immunologist, rheumatologist, general paediatrician. |
User tip
Editing a planned date on a treatment plan
Nurses and Medical Officers have the ability to edit a patient’s next planned treatment date on a treatment plan. This function ensures that electronic dose requests that are submitted to dispensing facilities contain the correct date information, which in turn ensures that the patient’s treatment intervals are updated correctly in BloodSTAR.
To edit a planned date follow the steps below:
1. Log into BloodSTAR as a Nurse or Medical Officer and locate the patient whose treatment plan you want to update.
2. Click on the patient’s authorisation reference number.
3. Scroll down to the Treatment Plan section and click on the pencil icon next to the next planned treatment date (see image below).
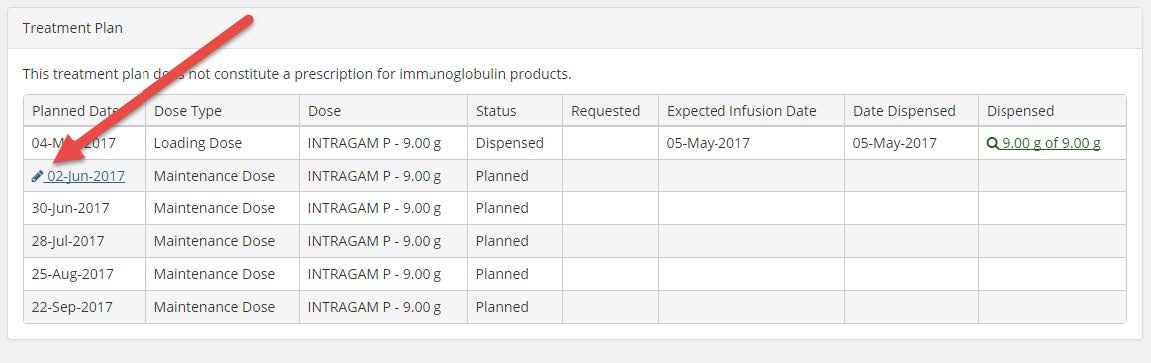
4. A pop up window will appear with the option to change the planned date. Type in the date required (DD/MM/YYYY) or click on the calendar icon and then select the date required on the calendar that appears. Click on the Save button.
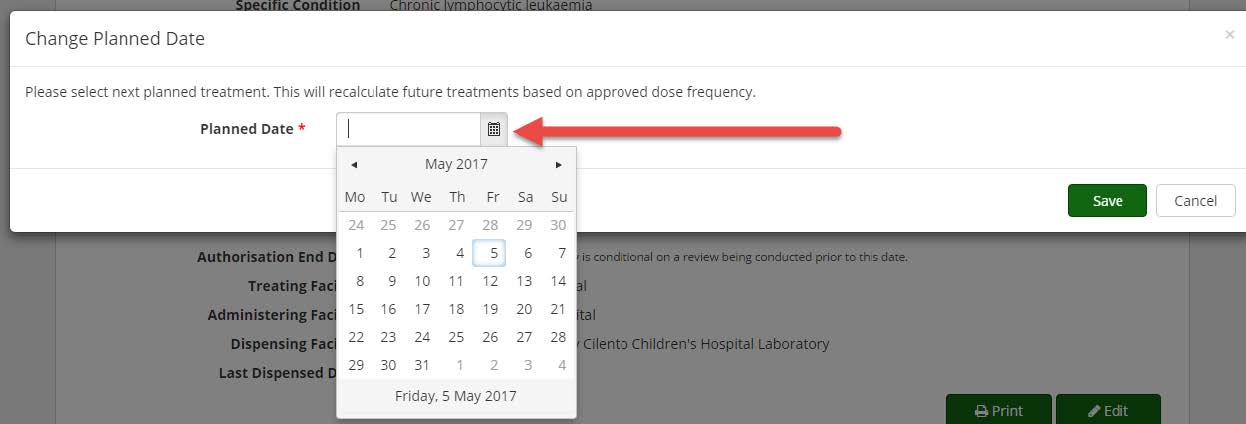
5. The treatment plan will update with the date you have selected. Please note, you can only move a date backwards a maximum of 28 days at a time.
Public consultation on changes to the Criteria
The public consultation process inviting comment on proposed changes to the Criteria for the Clinical Use of Intravenous Immunoglobulin in Australia (the Criteria) has recently closed. The revised Criteria have been developed by governments using expert Specialist Working Groups of clinicians to identify the medical conditions and circumstances for which immunoglobulin product is supplied and funded by all Australian governments under the national blood arrangements.
The recent consultation process supplements the consultation process undertaken in 2015 when the NBA sought feedback on conditions listed in Chapter 5 - Established Therapeutic Role and Chapter 6 – Emerging Therapeutic Role of the Criteria.
The recent public consultation process related to the proposed changes to access criteria for conditions listed in Chapter 7 - Exceptional circumstances only and Chapter 8 - Not supported. Also included in this round of public consultation are a small number of conditions listed in Emerging therapeutic role, where further proposed changes have been recommended since approval in 2015.
A small number of responses were received, and information regarding the responses and resulting changes will be published on the NBA website in due course.
More information about the criteria and public consultation documents can be found on the NBA’s website.
For further information
Further information on BloodSTAR is available online at www.blood.gov.au/bloodstar or by contacting the NBA on 13 000 BLOOD (13 000 25663) or support@blood.gov.au.
Previous editions
To read or download previous editions of BloodSTAR News, click on the relevant link below:

